Our Research
Our research centers on understanding how the brain can protect itself against neurological diseases (e.g., Alzheimer’s, Parkinson’s, stroke, multiple sclerosis, epilepsy, and migraine) and cognitive decline from aging. Brain is unique among organ structures. It can alter its regional, cellular, and molecular function as well as structure in response to activity. This is classically evidenced by Hebbian synaptic plasticity. However, it also extends to include environmental enrichment (i.e., increased intellectual, social, and physical activity), which protects the brain against neurological disease. Though incompletely defined, the mechanisms by which naturally increased brain activity strengthen brain involve immune signaling, the focus of our work.
Brain and immune functions are highly interactive and symbiotic, a relationship most often recognized in association with disease. For example, depressed mood reduces immune function and enhances susceptibility to disease. Also, increased immune function after disease onset worsens brain function. However, under physiological conditions, immune activation is nutritive when it is low-level and occurs over time, which allows for adaptation. This latter process is known as physiological conditioning hormesis. In fact, immune signaling is essential for the improved learning and memory associated with enrichment.
Our projects explore how enrichment triggers low-level pro-inflammatory immune signaling associated with oxidative stress which, over time, strengthens brain. We use whole animal and in vitro models. In addition, we use cellular and molecular imaging strategies as well as genomic and proteomic techniques and computational analyses of data from these approaches to search for the “signaling syntax” by which natural neural activity makes brain more resilient to disease. Our tools include time-lapse vital cellular imaging, laser dissection microscopy, real-time RT-PCR, semi-quantitative cellular cytological and immunohistochemical imaging, proteomic tools (including bead-based ELISAs), and novel neural therapeutic delivery systems.
Our hope is that the knowledge gleaned from these projects will empower healthcare providers and patients with evidence-based strategies that effectively enhance neurological health. Furthermore, this knowledge will help to develop novel therapeutics based on how the brain works best to preserve its health.
Specific research projects:
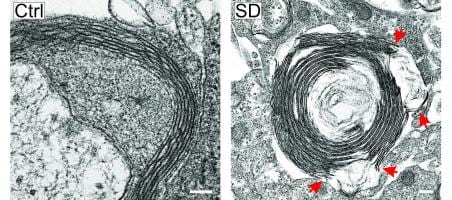
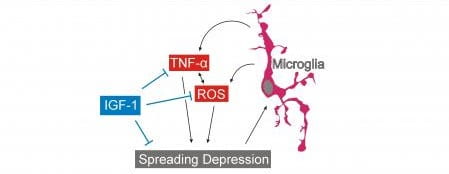
- The mechanisms and consequences of migraine, modeled via spreading depression
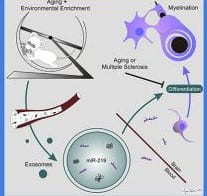
- The mechanisms by which enrichment results in neuroprotection
- Development of enrichment-based neural therapeutics